The CSI-360 is a “Controlled Substance Inventory System” designed to help optimize and automate some of the day-to-day operations in the veterinary practice. We took our pre-clinical offering, developed for the laboratory animal research market, and created an affordable solution for the effective management of controlled substances and other high value assets in the veterinary industry.
The CSI-360 was specifically designed to improve security, ensure compliance and facilitate the management of controlled substances in the veterinary clinic. Our solution focuses on diversion control, where other systems merely dispense the drugs. The CSI-360 combines a two-part authentication process, novel software and RFID tracking technology to provide secure access and 100% accurate recording of all controlled substance use in the veterinary practice. It is ideal for tracking high value assets, or items that require controlled access or strict inventory record, such as controlled drugs, surgical implants, laboratory reagents, supplies, and more. All records are captured electronically for a precise account of all cabinet access and activity, saving time and eliminating data entry errors.
Learn more about the CSI-360
Watch this short video for a product overview
The CSI-360 provides enhanced security features and paperless recording for efficient inventory management of controlled substances and other valuable items in the veterinary practice.
Increased Efficiency to Maximize Productivity
- Reduce manual labor with automatic and precise tracking and recordkeeping
- Eliminate data entry errors with paperless recording
- Optimize the appropriate inventory needed on hand
- Quick and easy access to inventory reports
Enhanced Security to Protect Your Practice
- Secure access with electronic lock and dual authentication to prevent unauthorized access
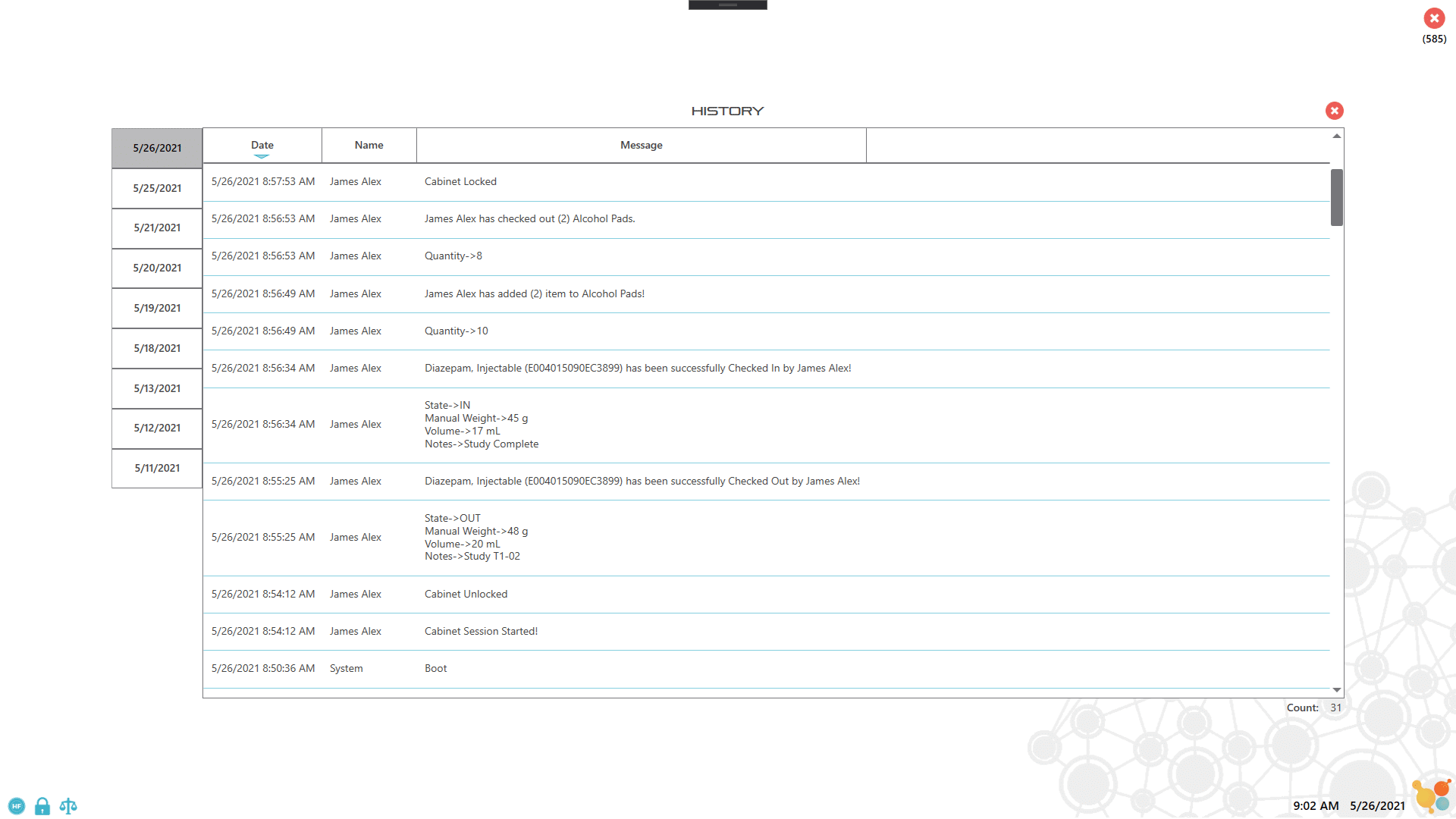
- Complete accountability of all cabinet activity, including shortages and misuse
- Accurate recordkeeping and audit trails to prevent theft, ensure compliance and avoid fines
Unique Capabilities
Peace of mind for veterinarians and their staff: prevent unauthorized access, theft, and drug misuse
System Specifications
Compatible with all cabinets, safes, refrigerators, and freezers from most manufacturers
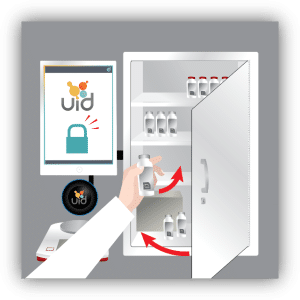
Our solution is designed to integrate with existing narcotics lock boxes to eliminate the painful process of DEA recertification. We provide an electronic locking mechanism, RFID technology for secure user access and asset identification (fob, key card or wrist band; RFID scanner and labels), a digital scale for precise measurements, a Windows Surface computer and inventory management software to enable efficient tracking and recordkeeping for controlled substances.
Simple Process. Easy to Use.
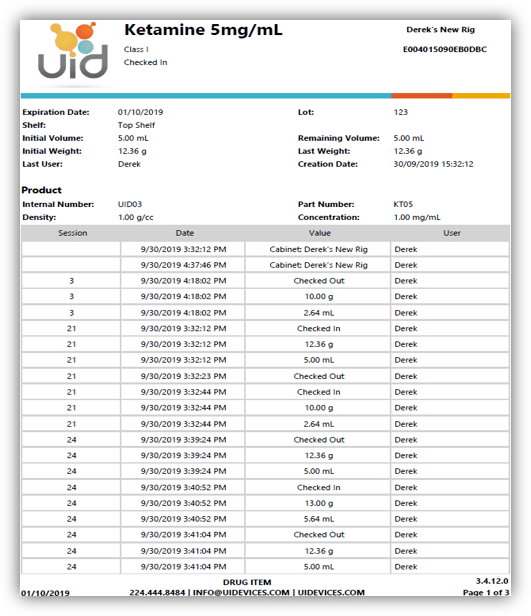
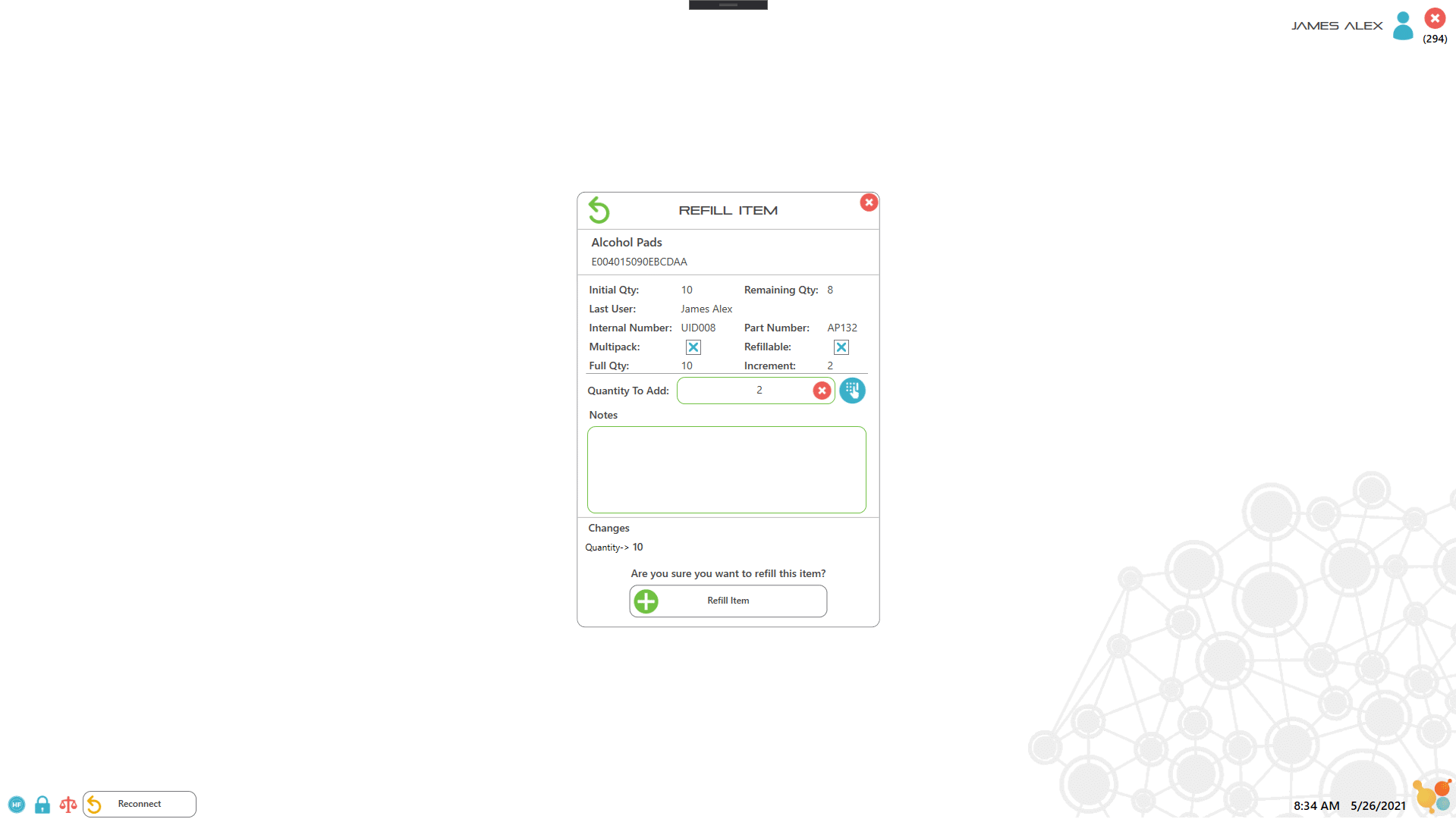
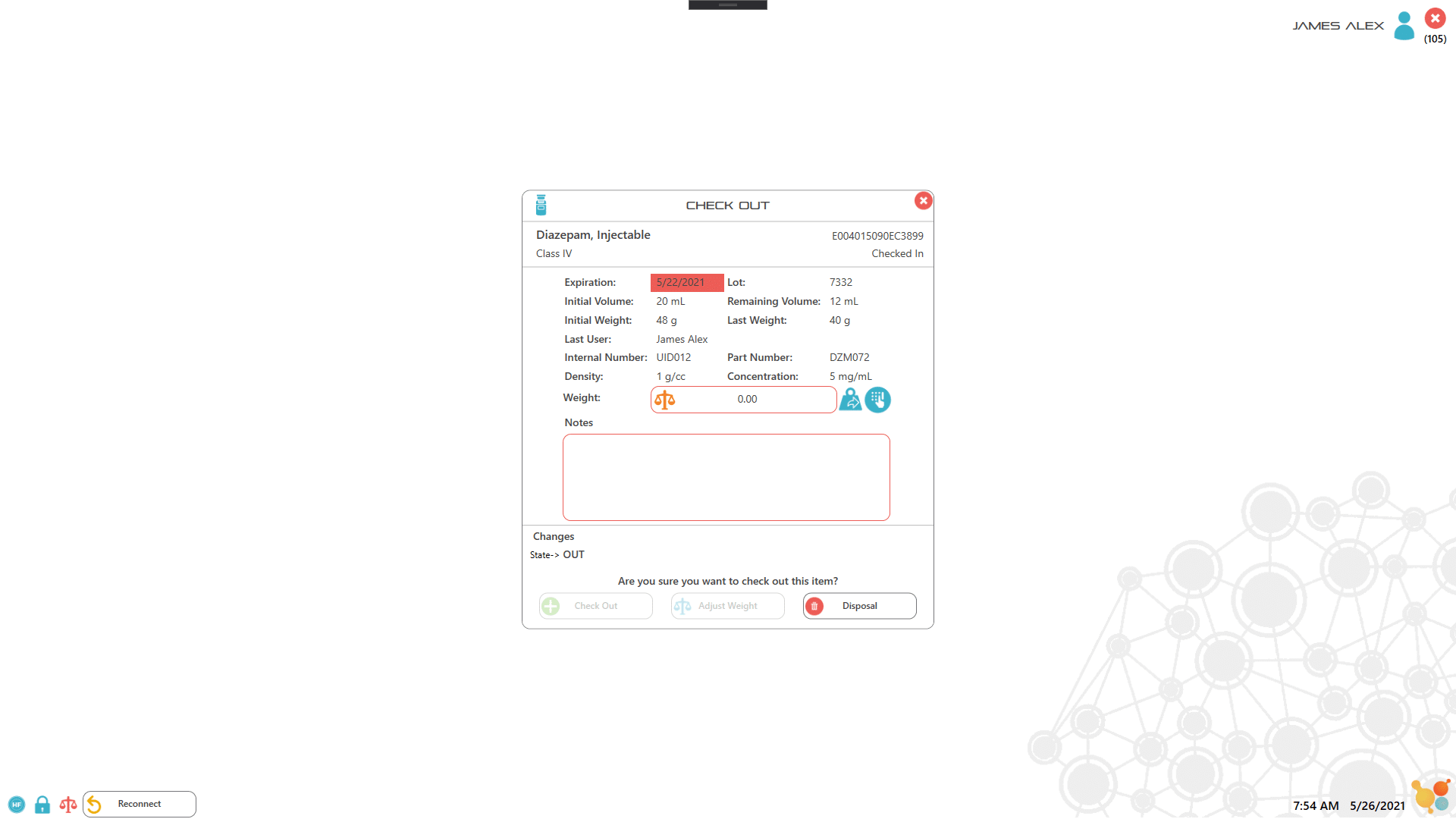
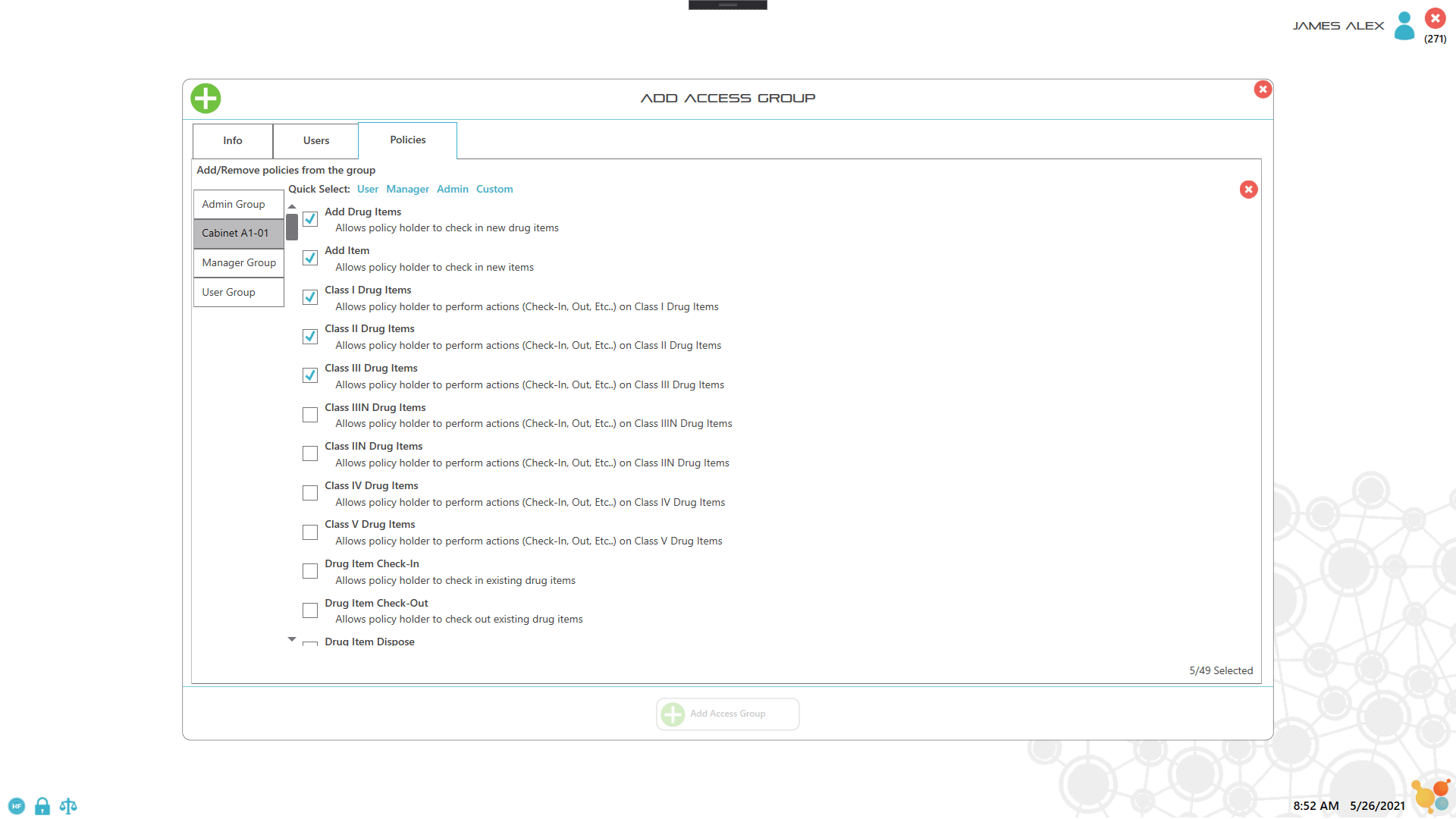
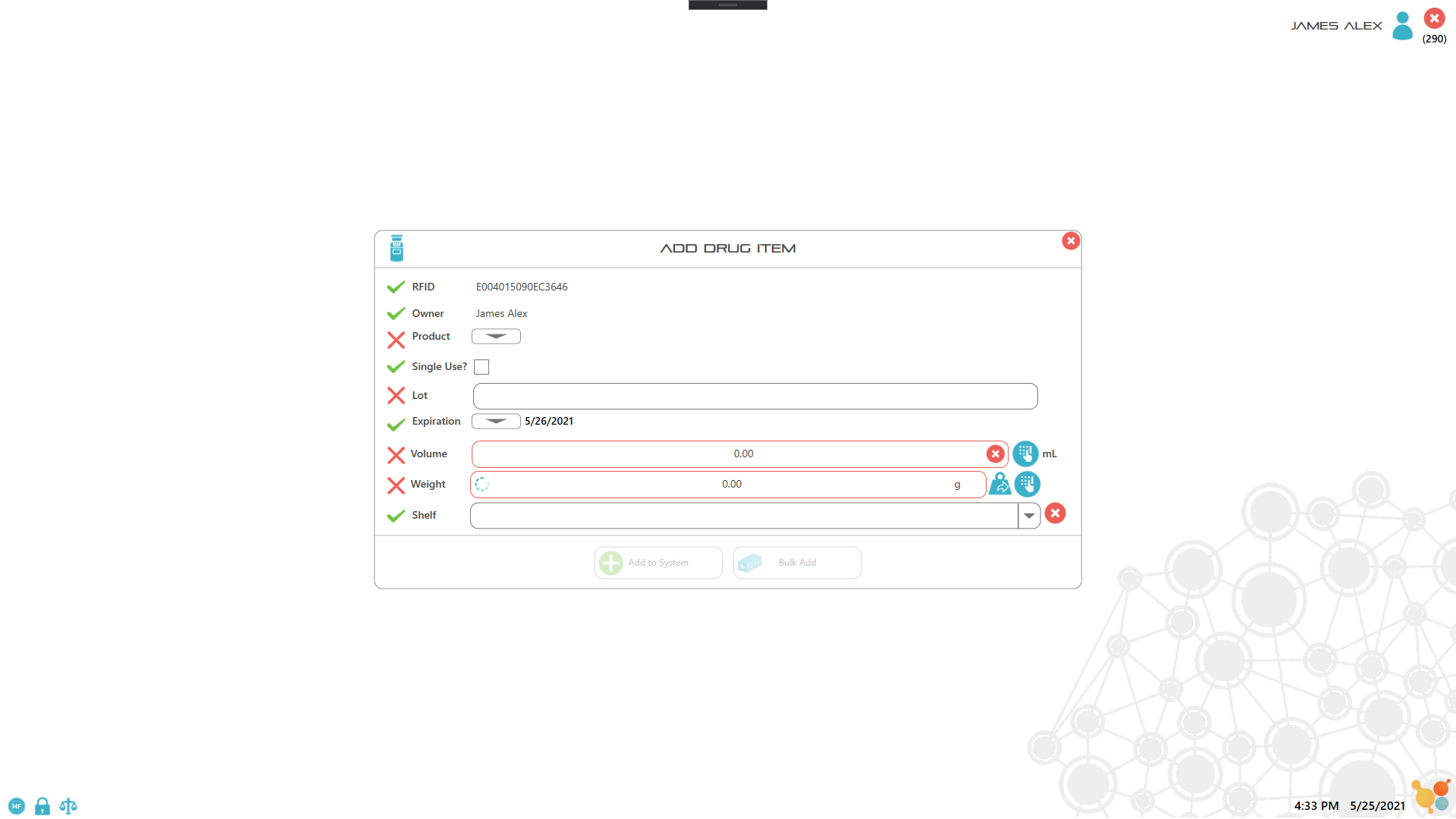
Each item or drug container is labeled with an RFID tag which the user scans to record key information about the item. For assets such as controlled drugs, the process of recording drug use is controlled by scanning the RFID labels and either weighing the container, or manually entering the amount used. All records are captured electronically to ensure 100% accuracy of drug inventory, as well as to facilitate documentation and reporting. Extra security access is implemented by combining lock or key access with RFID user badges with pictures and PIN codes, which ensure 100% accurate entry/exit recording.
1. Label asset and user
2. Secure login with two-factor validation
3. Remove, scan and weigh the item
4. Record notes
5. Use item and weigh to record used amount
6. Return item and close door
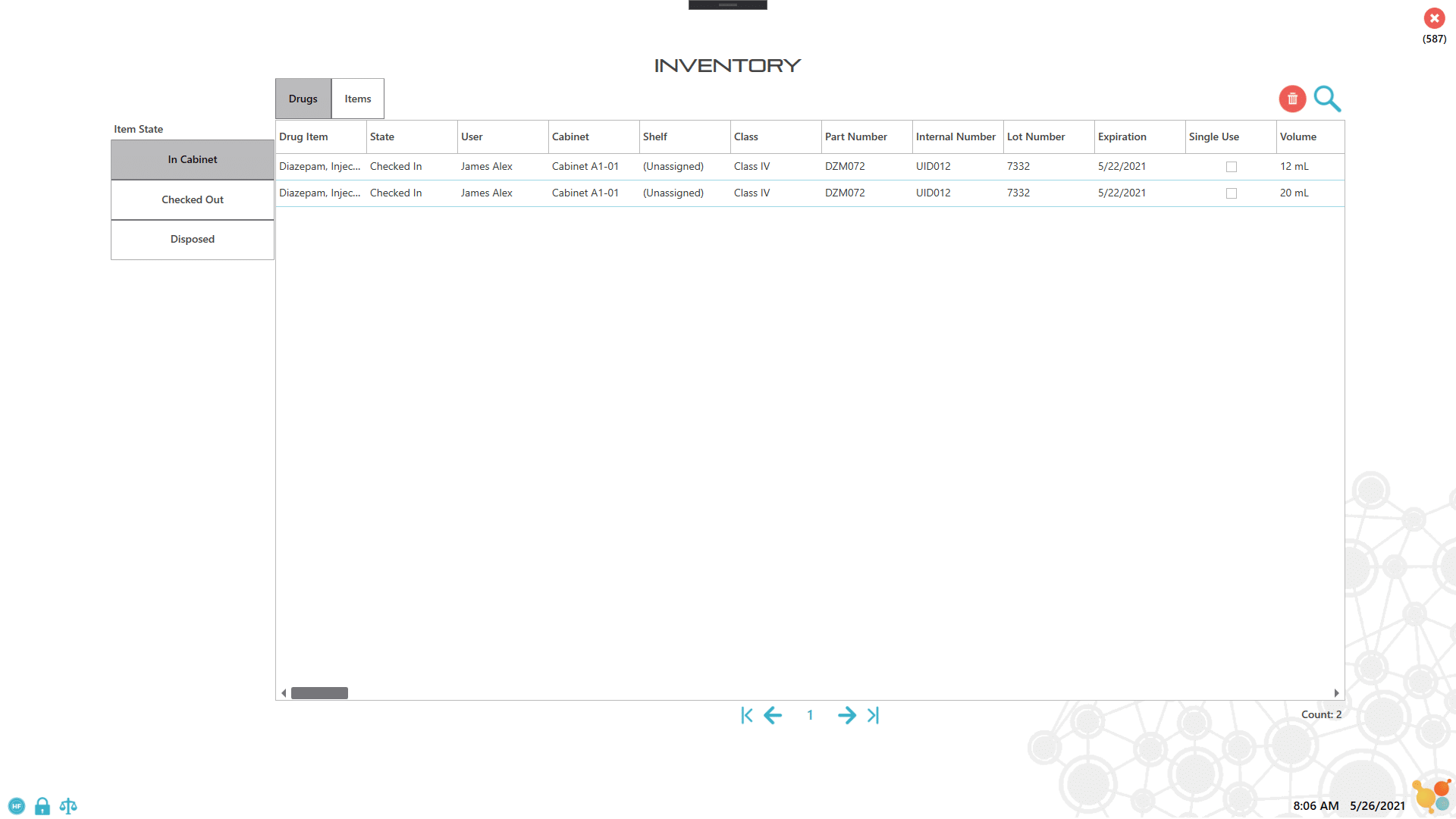
Paperless Recording and Easy Access to Inventory Reports
Most veterinary facilities store controlled substances in a combination-lock safe or cabinet with a one- or two-key security system in place that can only be accessed by authorized personnel. The use and record-keeping process relies entirely on manual data entry, which is labor-intensive and prone to miscalculations and human errors. The CSI-360 provides enhanced security features and paperless recording to facilitate the inventory management process for controlled substances. Our solutions eliminate manual data entry errors and reduce the amount of manual labor needed for tracking DEA logs, inventory logs and supplies by providing automatic inventory tracking capabilities. Reports for all cabinet activity, such as user access, drug amount or volume used, or times of access are all easily retrievable and readily available for DEA audits or other needs.
Managing Controlled Substances doesn’t have to be a daunting task!
New Kiosk Design
UID partnered with Global American to develop a custom Mini-ITX motherboard assembly tailored for a compact enclosure. Key requirements included low-power consumption, long-term reliability, customizable power connections, and cost-effective memory and storage. Leveraging their expertise, Global American delivered a solution that met all specifications, resulting in a user-friendly system designed for efficient controlled substance management in animal research facilities.